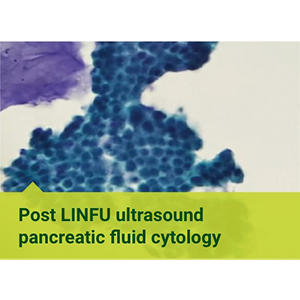
A proven approach
Quest is pleased to be part of a nationwide clinical registry which is collecting long term patient outcome data on a “Pap smear for the pancreas”™ approach of using screening cytology to aid in the early detection and prevention of pancreatic cancer.
In the 1950’s, cervical cancer was among the largest causes of cancer death in American women. Almost everyone had a mother, sister, aunt, niece, wife, or daughter who died from the disease. By the 1970’s however, US cervical cancer deaths had dropped to 14, and remain uncommon today.